Normax FAQ
mRNA vaccine technology is a transformative new sector of the biopharmaceutical industry which has revolutionized vaccine development and is helping to end the COVID-19 pandemic
23 frequently asked questions and answers are here…
Our mission is to:
- drive down the cost of mRNA Vaccines to save more lives and to deliver sustainable returns for impact investors.
- deliver safe and effective mRNA vaccines for infectious diseases, at large scale, for about $4 dollars per dose, on advance purchase agreements.
- finance and deliver up to 100 novel mRNA Vaccine products and up to 1oo mRNA Vax Factories worldwide.
- participate as a strategic partner in the global ecosystem for transformative research, development and manufacture of safe and effective mRNA vaccines for global public health and pandemic preparedness and prevention, with competitive financial performance.
We believe our mission is honorable and achievable.
WHY?
Our Purpose is to deliver safe, effective and affordable mRNA Vaccines to save lives.
- Help Enable Pandemic Preparedness
- Support Global Public Health
- Help Prevent and Cure Disease
- Save Lives and Prevent Human Misery
- Help to prevent future Pandemics
HOW?
Safe and effective mRNA Vaccines are delivered through sustained process with zero error and:
- People, Partners and Knowledge
- Leading Science and Technology
- Private and Public Impact Investment
- Advance Purchase Agreements (APA)
- Vaccine Manufacturing in modular GMP Vax Factories
WHAT?
Normax delivers collective results with affordable mRNA Vaccines for all.
- mRNA Vaccines for Disease and Cancers
- Vaccine Manufacturing and Fill-Finish
- mRNA Vaccine Platform
- mRNA Vaccines for Public Health
- Support Pandemic Preparedness for all
Persistent. Passionate. Progressive. Productive. Personal.
Persistent. We are innovative and relentless in our battle against pathogens and disease.
Passionate. We build strong and diverse teams with passion for success.
Progressive. We think and act like stakeholders with pride of ownership.
Productive. We are smart, effective, talented, collaborative, persistent.
Personal. We strive for our customers to love us and our mRNA Vaccine products.
Our long-term growth goal is to develop up to 100 mRNA Vaccines and deploy up to 100 Vax Factories around the world.
1. mRNA Vaccine R&D
Normax conducts mRNA Vaccine R&D with a standardized mRNA vaccine technology in cooperation with leading scientist and researchers around the world.
2. mRNA Vaccine Manufacturing
mRNA Vaccine Manufacturing with modular GMP regulatory complaint Vax Factory in cooperation with mRNA UCV Consortium of Vendors and Partners.
Normax estimates that the average cost for Normax Vax Factory to manufacture mRNA Vaccines at large scale for infectious diseases is about $2.00 per dose, or less.
Normax plans to sell our mRNA Vaccines for infectious diseases on large scale advance purchase agreements (APA) with customers at about $4.00 per dose.
This represents a gross profit on sales of 100%.
While big pharma charges anywhere from $8 to $35 per COVID vaccine dose, Normax believes that a 100% gross profit mark-up on sales represents a sustainable and profitable high-impact business for our investors.
The first mRNA Vaccines currently in early stage research and development by Normax are for:
- SARS-CoV-2 with a “Universal Covid Vaccine” (UCV)
- Tuberculosis (TB)
- Human Immunodeficiency Virus (HIV)
- Malaria
In theory, mRNA vaccines by Normax could save-a-life for less than the cost of a hamburger.
mRNA vaccine technology is a transformative new sector of the biopharmaceutical industry which has revolutionised vaccine development through increased speed, safety and cost-effective development leading to the first approved vaccines for the COVID-19 pandemic.
How mRNA Vaccines Work
- mRNA technology allows researchers to fast-track the early stages of vaccine research and development and thereby to produce vaccines faster and more efficiently.
- Scientists generate an mRNA sequence that codes for a protein target on the virus.
- The mRNA sequence of base pairs is a molecular blueprint for cells to produce a targeted protein that stimulates a potent immune response.
- The mRNA vaccine delivery mechanism is using advanced Lipid nanoparticle technology which is a sophisticated and innovative non-viral gene delivery technology.
How mRNA Vaccines will be Manufactured in Vax Factory by Normax
- Step 1: mRNA Synthesis
- Step 2: mRNA Purification
- Step 3: mRNA Encapsulation in Lipid Nanoparticles
- Step 4: Vaccine Fill and Finish into Glass Vials
- Step 5: Vaccine Packaging and Delivery
- For pandemic preparedness, the freeze-drying of vaccines and long-term stockpiling at ambient temperature is expected to be technically possible in the future.
How mRNA Vaccines are Used
- Step 1: The mRNA Vaccine is clinically assessed for safety and efficacy.
- Step 2: If found to be safe and efficacious, the vaccine is approved by Regulatory Authorities
- Step 3: The mRNA Vaccine is injected into the patient
- Step 4: The mRNA instructions for producing proteins are decoded in the cell to deliver millions of copies of simulated viral protein fragments into the bloodstream.
- Step 5: These protein fragments stimulate the natural immune system to produce antibodies that will protect the patient when a real virus enters the body.
How mRNA Vaccines are monitored for Safety and Effectiveness
- Following regulatory approval, safety and effectiveness are continuously monitored.
- As new virus variants emerge, the existing vaccine can be modified with a newly programmed mRNA sequence in a matter of hours and a newly updated mRNA vaccine could be developed in 6 weeks. This would then undergo safety and efficacy assessment prior to regulatory approval.
With an early stage budget of €25,000,000 each, Normax is currently developing mRNA Vax Factories planned to be located in:
- Ireland
- Switzerland
- The United Kingdome
- The United States
- Open Allocation
It takes 18-24 months from launch to deliver and deploy a regulatory compliabnt and productive Vax Factory by Normax.
Vaxcomm is The Vaccine Freedom to Operate (FTO) Commons
Our Focus: mRNA technology applied to infectious disease, treatments, cancers, global public health and pandemic preparedness.
Vaxcomm is a Global Public Health Commons with Open Participation and Membership Opportunities for Scientists, R&D Organizations and Industry.
The mission of Vaxcomm is:
- To deliver advanced Vaccine Technology Commons for benefit of Global Public Health and members
- To deliver transformative mRNA technology opportunities with shared knowledge and commons licensed intellectual property (IP) according to community managed rules
- To advance mRNA vaccine technology for the benefit of Vaxcomm members and global public health
Normax is in the business of mRNA vaccine Research, Development and Manufacturing.
- Normax has secured a €300,000,000 capital commitment from a $3.4Bn cornerstone institutional investor for development of mRNA Vaccines and Vax Factory Manufacturing for Transformative Social Impact on Infectious Disease and Pandemic Preparedness.
- Normax plans to drive down the cost of mRNA Vaccines to save more lives and to deliver sustainable returns for impact investors.
- Normax plans to deliver safe and effective mRNA vaccines at large scale for about $4 dollars per dose.
- Normax mRNA vaccine products in development include: (1) mRNA Vax Factory, (2) Universal Coronavirus mRNA Vaccine, (3) Tuberculosis mRNA Vaccine, (4) HIV mRNA Vaccine, (5) Malaria mRNA Vaccine, and (6) Disease-X mRNA Vaccine (e.g. within 100 days).
- Normax Biomed Ltd (Normax) is based in Cork, Ireland, and London, United Kingdom.
Normax mission is to deliver competitive financial performance with transformative social impact.
NOT AN OFFER TO INVEST.
Abstract. The SARS-CoV-2 pandemic has generated a renaissance in vaccinology, with COVID-19 mRNA vaccines delivering a “digital code” of the viral antigen with no need to purify proteins or inactivate pathogens.
Vaccines 2020: The era of the digital vaccine is here
Mariagrazia Pizza, Simone Pecetta, Rino Rappuoli
Science Translational Medicine
15 Dec 2021, Vol 13, Issue 624, DOI: 10.1126/scitranslmed.abm3249
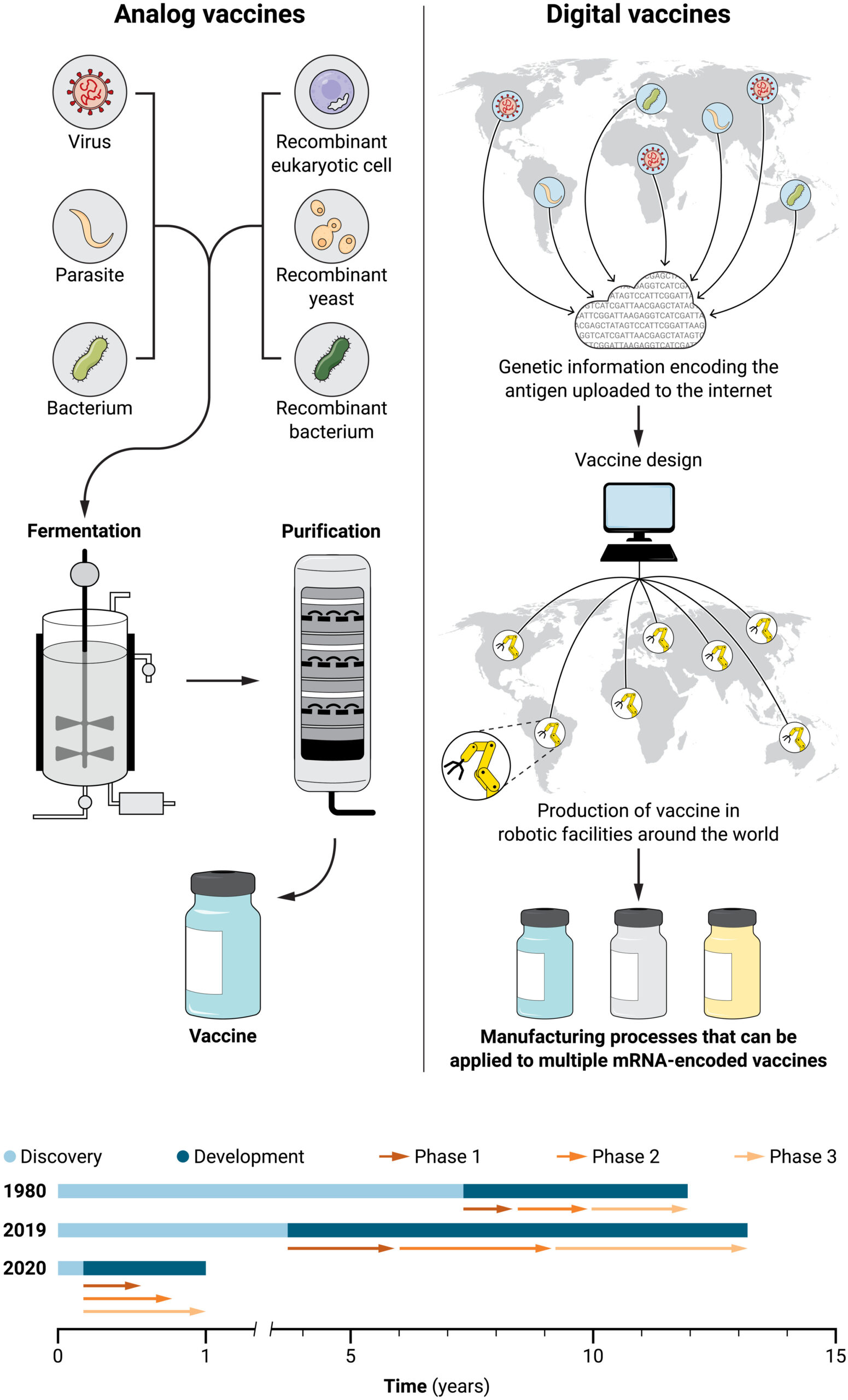
Fig. 1. Impact of digital vaccines on vaccinology.
Analog vaccines (left) require isolation of the pathogen or the generation of a recombinant cell line to produce the protein antigen through fermentation and purification. This is a complex and long process where scale-up of new manufacturing methods can take years. In contrast, digital vaccines (right) only require genetic information encoding the antigen and not the protein antigen itself. Such genetic information can be shared via the internet and used by multiple laboratories and production facilities around the world. In the case of mRNA-encoded vaccines, the same manufacturing process and facility can be used for multiple vaccines. Digital vaccines enable much faster vaccine development (bottom). Traditional analog vaccines can take 10 to 15 years from discovery to clinical use. Parallel clinical development of COVID-19 mRNA vaccines, with overlap of phase 1, 2, and 3 clinical trials, resulted in emergency use authorization by the FDA within 10 months, without compromising safety and efficacy.
Normax Press Release
Normax secures €300,000,000 capital commitment from Global Emerging Markets (GEM) for research and development and manufacturing of mRNA Vaccines for transformative global social impact
Normax plans to use capital finance to scale mRNA Vaccine Research and Development with Vax Factory Manufacturing for Transformative Social Impact on Infectious Diseases, Rare Diseases, Cancers and Pandemic Preparedness
July 11, 2022, 12:00 PM Greenwich Mean Time (GMT) – PRESS RELEASE. NOT AN OFFER. SEE DISCLAIMER.
DUBLIN, Ireland — The mRNA vaccine technology company Normax Biomed Limited (“Normax”), today announced a €300 Million investment commitment from GEM Global Yield LLC SCS (“GEM”), the $3.4 Billion, Luxembourg based, private alternative investment group that focuses on emerging markets with offices in Paris, Nassau (Bahamas) and New York.
Under the agreement, GEM will provide Normax with a Share Subscription Facility (“SSF”) of up to €50 Million for a 36 month term following the public listing of the Normax common stock, and an SSF of up to €250 Million for up to ten specialized Normax Subsidiary Companies (“SPV”) of Normax Group. Normax will control the timing and maximum amount of each drawdown under the facility and has no minimum subscription obligation. Normax plans to list on SIX Swiss Exchange and/or SIX Digital Exchange (SDX) and/or London Stock Exchange with digital Normax mRNA Vaccine and Vax Factory SPV listings on SDX.
The SSF entered into with GEM Global Yield LLC SCS, Luxembourg, and GEM Yield Bahamas Ltd (collectively “GEM”) is a EUR 300 million facility over three (3) years and allows Normax to draw down funds at its option in exchange for Normax common shares. The mechanics of the GEM facility allow for multiple draw-downs, the amount being in a range related to the trading volume and price of the Normax shares. Normax will control the timing and maximum amount of any draw-down, and has the right, not the obligation, to draw down on the full committed amount. The draw-down amount is based on 90% of the average closing price and the average volume of the Normax common shares of the last 15 trading days. The consideration for the GEM facility is due for payment by Normax in twelve months from the listing of the shares on SIX Swiss Exchange and can be paid by way of Normax shares. In addition, GEM has been granted warrants to purchase Normax shares.
Leading mRNA Biotech Companies (Public Industry Reference Data)
TICKER MARKET CAP LINK
- MRNA $50+ Billion Moderna, Inc.
- BNTX $30+ Billion BioNTech SE
- ALNY $20+ Billion Alnylam Pharmaceuticals, Inc.
- SRPT $9+ Billion Sarepta Therapeutics, Inc.
- IONS $5+ Billion Ionis Pharmaceuticals, Inc.
- ARWR $4+ Billion Arrowhead Pharmaceuticals, Inc.
- CVAC $1.5+ Billion CureVac N.V.
- ARCT $0.4+ Billion Arcturus Therapeutics Holdings Inc.
Private Companies:
- Abogen Biosciences $817 million
- Laronde $490 million
- Normax Biomed $300 million
- Sirnaomics $270 million
- Deep Genomics $236 million
- Nutcracker Therapeutics $219 million
- VaxEquity $195 million (ICL and AstraZeneca)
- eTheRNA $60 million
- DIOSynVax $42 million (Univ. of Cambridge, GOV UK and CEPI)
- Ethris $26 million
Big Pharma active in mRNA vaccine therapeutics R&D
- AstraZeneca
- GSK
- Janssen/Johnson & Johnson
- Merck KGaA
- Merck/MSD
- Novartis
- Pfizer
- Roche
- Sanofi ($3.2 billion acquisition of Translate Bio)
Simple answer:
- Nature is fundmentally not patentable. Nature is not a “monopoly business”
- DNA translated into mRNA and expressed as Proteins, in the cell, is the basis for all life on earth.
- Of about 10,000 diseases in humans, only 500 are currently treatable.
- mRNA technology-based products can be safe and effective for many diseases and conditions.
- Normax is an mRNA Freedom To Operate (FTO) business.
- Generally, big-pharma are in the “monopoly” business, but in practice, no single company can monopolize the basic mRNA business.
- Normax plans to compete – on price – with safe and effective regulatory approved products, for a relatively unlimited global healthcare market demand/need.
- The “IP landscape” must to be managed for FTO of the business – and it is this strategic FTO capability that is expected to be a competitive advantage for Normax.
Normax has engaged Berkeley Communications an international full-service PR and communications agency.
+44 118 909 0909
