STATISTICS AND SAFETY. 13.26 billion Coronavirus (COVID-19) Vaccinations. (668 million in U.S.) COVID-19 Vaccine Safety Surveillance – FDA and CDC
Coronavirus (COVID-19) Vaccinations – Our World in Data
-
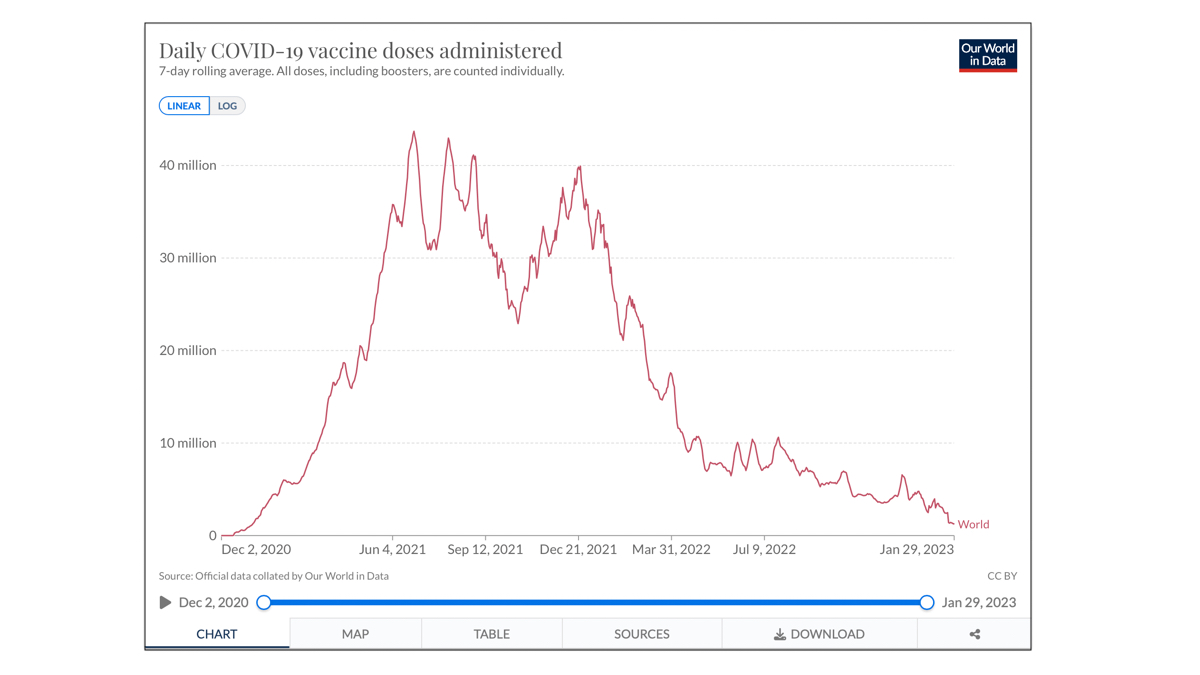
69.4% of the world population has received at least one dose of a COVID-19 vaccine.
-
13.26 billion doses have been administered globally, and 1.27 million are now administered each day.
-
26.4% of people in low-income countries have received at least one dose.
COVID-19 Vaccine Safety Surveillance – FDA
Summaries of Monitoring Efforts
FDA is conducting intensive monitoring of COVID-19 vaccine safety in the U.S. using a variety of approaches. Based on available information, FDA strongly believes that the known and potential benefits of COVID-19 vaccination greatly outweigh their known and potential risks. As part of our efforts to be transparent about our COVID-19 vaccine safety monitoring activities, FDA is posting summaries of the key safety monitoring findings on this page.
- Initial Results of Near Real-Time Safety Monitoring of COVID-19 Vaccines in Persons Aged 65 Years and Older – July 9, 2021
- CDC and FDA Identify Preliminary COVID-19 Vaccine Safety Signal for Persons Aged 65 Years and Older – January 13, 2023
More than 668 million doses of COVID-19 vaccine have been given in the United States from December 14, 2020, through January 26, 2023. To view the current total number of COVID-19 vaccinations that have been administered in the United States, please visit the CDC COVID Data Tracker.
COVID-19 vaccines are safe and effective. COVID-19 vaccines were evaluated in tens of thousands of participants in clinical trials. The vaccines met the Food and Drug Administration’s (FDA’s) rigorous scientific standards for safety, effectiveness, and manufacturing quality needed to support emergency use authorization (EUA). Learn more about EUAs in this video.
The Pfizer-BioNTech, Moderna, and Johnson & Johnson/Janssen and Novavax COVID-19 vaccines will continue to undergo the most intensive safety monitoring in US history. This monitoring includes using both established and new safety monitoring systems to make sure that COVID-19 vaccines are safe.
Common Side Effects
Some people have side effects after getting their COVID-19 vaccine, while others might have no side effects. Side effects may affect the ability to do daily activities, but they should go away within a few days. Learn more about common side effects after COVID-19 vaccination.
Adverse Events (Serious Safety Problems) Are Rare
In rare cases, people have experienced serious health events after COVID-19 vaccination. Any health problem that happens after vaccination is considered an adverse event. An adverse event can be caused by the vaccine or can be caused by a coincidental event not related to the vaccine, such as an unrelated fever, that happened following vaccination.
To date, the systems in place to monitor the safety of these vaccines have found four serious types of adverse events following COVID-19 vaccination, with evidence that suggests, although rare, a link to certain types of COVID-19 vaccinations that were administered. They are:
Anaphylaxis
Anaphylaxis is a severe type of allergic reaction with symptoms such as hives, difficulty breathing, low blood pressure, or significant swelling of the tongue or lips. Anaphylaxis after COVID-19 vaccination is rare. Learn more about COVID-19 vaccines and allergic reactions, including anaphylaxis.
Thrombosis with Thrombocytopenia Syndrome (TTS)
Thrombosis with thrombocytopenia syndrome (TTS) is a rare but serious adverse event that causes blood clots or issues with clotting. TTS after COVID-19 vaccination is rare. Learn more about COVID-19 vaccines and adverse events, including TTS.
Myocarditis and Pericarditis
Myocarditis is inflammation of the heart muscle, and pericarditis is inflammation of the outer lining of the heart. Myocarditis and pericarditis after COVID-19 vaccination are rare. Learn more about COVID-19 vaccines and adverse events, including myocarditis and pericarditis.
Guillain-Barré Syndrome (GBS)
Guillain-Barré Syndrome (GBS) is a rare disorder where the body’s immune system damages nerve cells, causing muscle weakness and sometimes paralysis. GBS after COVID-19 vaccination is rare. Learn more about COVID-19 vaccines and adverse events, including GBS.
Reports of Death Are Rare
Reports of death after COVID-19 vaccination are rare. FDA requires healthcare providers to report any death after COVID-19 vaccination to the Vaccine Adverse Event Reporting System (VAERS), even if it’s unclear whether the vaccine was the cause. Reports of adverse events to VAERS following vaccination, including deaths, do not necessarily mean that a vaccine caused a health problem. CDC and FDA review reports of death following COVID-19 vaccination and update information as it becomes available. Learn more about adverse events, including reports of death, after COVID-19 vaccination.
Benefits of Vaccination Outweigh the Risks
Serious side effects that could cause a long-term health problem are extremely rare following any vaccination, including COVID-19 vaccination. The benefits of COVID-19 vaccination outweigh the known and potential risks.
CDC continues to closely monitor the safety of COVID-19 vaccines. Everyone who receives a COVID-19 vaccine can also participate in safety monitoring by enrolling themselves -or their children or dependents 6 months and older in a smartphone-based system called v-safe and completing health check-ins after COVID-19 vaccination.
Have you experienced a side effect following COVID-19 vaccination?
Please report it to VAERS. In addition, enrolling yourself or your dependent in v-safe allows you to easily report to CDC how you are feeling after getting a COVID-19 vaccine.
More Information
- ACIP COVID-19 Vaccines Safety Technical Sub-Group (VaST)
- COVID-19 Vaccine Safety Publications
- VaST Subgroup Technical Report
- v-safe After Vaccination Health Checker
- Vaccine Adverse Event Reporting System (VAERS)
The Vaccine Adverse Event Reporting System (VAERS)
DISCLAIMER EXAMPLE…
The Vaccine Adverse Event Reporting System (VAERS)
The Vaccine Adverse Event Reporting System (VAERS) database contains information on unverified reports of adverse events (illnesses, health problems and/or symptoms) following immunization with US-licensed vaccines. Reports are accepted from anyone and can be submitted electronically at www.vaers.hhs.gov.
EXAMPLE…
| Search Current VAERS Data
The information in this database contains reports received from 1990 to the present. Data can be searched by the following: age, event category, gender, manufacturers, onset interval, recovery status, serious/non-serious category, state/territory, symptoms, vaccine, VAERS ID #, year reported, month reported, year vaccinated and month vaccinated. Click the VAERS Data Search button below to begin your data search. * This allows you to search for details on a specific VAERS report by the VAERS ID number. |
How to Access Data from CDC’s VAERS WONDER System
Written steps: How to access VAERS data
Video: How to access VAERS data
This video demonstrates how to search VAERS data using CDC WONDER. You will also learn about the purpose of VAERS and strengths and limitations of VAERS data.
Watch specific sections of the video:
- Section 1: Introduction to VAERS
- Section 2: How to Search VAERS Public Data
- Section 3: Strengths and Limitations of VAERS Data
- Section 4: Where to Get More Information
Learn More
-
THE LANCET. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study
-
CDC. COVID-19 Vaccines Work
-
WHO. A Brief History of Vaccination